THE EFFECT OF ENVIRONMENT ON CORROSION
ABSTRACT
In this research project, the various nail was subjected to six environments so as to check the effect of the environments on corrosion of the nails. Two sets of nails were used; normal nail and concrete nail. Six of each type while the other not coated. The research was carried out in the physical chemistry laboratory of the institute of management and technology. The environments used are the acidic solution, alkaline solution, salt solution, boiled water, air medium, and lubricating oil medium… (Scroll down for the link to get the Complete Project Material)

INTRODUCTION
In its broadest sense, the term corrosion applied to the destructive alteration of a metal or alloy by chemical reaction with any substance, solid, liquid, or gas. The pattern of attack will be governed largely by the combined influence of several factors relating to the metal or alloy, to the conditions of service, and to the chemical nature of the environment.
- The metal may corrode uniformly over its entire surface, as in the resting of iron in the atmosphere.
- It may suffer only a superficial attack that does not seriously affect the strength of the metal or alloy but does discolor the surface, as in the tarnishing of silver in the atmosphere.
- Corrosion may develop at local areas on an otherwise unattached surface, leading to pitting that may in some instances result in early failure by perforation.
- An alloy may be attacked in such a way as to remove one or more of its constituents, leaving a weakened residue of an unattached element with poor metallic properties, as in the dezincification of yellow brass or in the graphitization of cast iron.
- Excessive corrosion may occur in local areas where poor design features tend to aggravate the corrosion conditions, as crevices under rivets, bolts, and faying surface.
- The use of dissimilar metals sometimes can lead to a severe attack of the more active metal where they are joined together.
- High tensile stresses plus specific corrosive conditions may result in cracking of a metal because of a corrosion reaction along critical paths in the metal.
In order to provide a quantitative basis for reporting corrosion rates and intensity of the attack, the average weight loss of metal per unit area per unit time or the loss in thickness per unit time is usually recorded… (Scroll down for the link to get the Complete Project Material)
Research Objectives
The objective of this study is based on the fault that corrosion which has been a major problem in our modern industries has to be prevented and curbed to the barest minimum by putting its environment into consideration in selecting material a structure or piece equipment.
Significance of the Research
A complete understanding of corrosion phenomena in all fact requires intensive and prolonged study. However, general knowledge of the subject can be gained by the engineer through a review or survey of the various ways in which corrosion manifest itself, the factors that govern the corrosion process, and the means available to the engineer for controlling or preventing corrosion… (Scroll down for the link to get the Complete Project Material)

LITERATURE REVIEW
There are several environmental factors that influence Steel materials exposed to the atmosphere. The degree of impact of these factors on the exposed materials depends on the intensity and concentration of these factors at each location and can also be a combined effect of interrelated environmental factors on the Steel.
Effect of Sea Water Salt on the Corrosion of Corrosion of Steel
Chloride ions are often found in industrial areas and exist as one of the most vital and common atmospheric corrosive agents [16, 17]. The presence of the chloride ions in the atmosphere can influence the corrosion of the Steel pipeline exposed to atmospheric conditions and may lead to the failure of the entire pipeline system [18-20].
In the atmospheric corrosion process of the Steel pipeline, corrosion is initiated by droplets of atmospheric water mostly from the seashore, coupled with residual stress imposed on the pipeline system due to the transportation of petroleum products, which often results in chloride-induced SCC [21]. Several studies have been carried out on the corrosion behavior of different steel materials exposed in the chloride environment [22-24]… (Scroll down for the link to get the Complete Project Material)
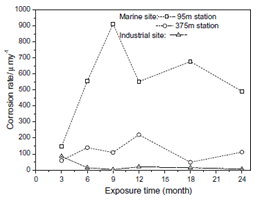
Figure 1: Corrosion Rate of Steel in Different Atmosphere Conditions [5]
On examination of the steel sample, the result revealed that Cl_ ion influenced the corrosion rate, morphology, and composition of the Steel material as shown in Figure 1.
Sea salt water is commonly used in many applications as sodium chloride and calcium chloride and has been dated back to the early centuries. The usage has an associated amount of damage to some structures existing within the atmospheric environment as reported by several researchers [25-28]. Sea salt has been reported as one of the predominant environmental contaminants that corrode the Steel pipeline especially in the coastal areas where the sea salt concentration is relatively high [29]… (Scroll down for the link to get the Complete Project Material)
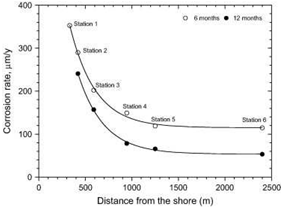
Figure 2: Variation in The Corrosion Rate of Steel With Distance from the Shore [31]
Other researchers have also shown that the sea-salt contains some quantities of magnesium chloride and sodium chlorides which corrode the pipeline Steel at low humidity [33, 34]. Ericsson [34] revealed that a significant quantity of SO2 is contained in sea salt water and can as well aggravate the corrosion rate of Steel. Another result from his study showed that the combination of SO2 and sea salt water increased the corrosion rate several times more than the contribution of individual constituent [34]… (Scroll down for the link to get the Complete Project Material)
Effect if Sulphide Dioxide on Corrosion of Steel Materials
Sulfur dioxide (SO2) in addition to other environmental pollutants is one of the dominant constituents affecting the corrosion rates of Steel exposed to atmospheric conditions [35-37]. Misawa et al.[38] attempted to explain the basis of electrochemistry of sulfur dioxide and corrosion of steel materials in 1974. The effort of understanding the mechanism of atmospheric corrosion of steel materials was supported by group researchers [37] in 1997, where the aggressiveness of SO2 on Steel materials was explored and detailed.
Several studies have been conducted on the effect of atmospheric SO2 pollution on steel materials [7, 39, 40]. Leygraf et al. [41] reported that a significant amount of SO2 deposited on the surface of Steel can result in the formation of the protective layer. The result further revealed that an increased amount of SO2 can cause acidification of the aqueous layer, resulting in material anodic dissolution [41].
Other researchers have shown that the corrosion of steel materials due to the presence of SO2 is an integral contribution of other parameters [42]… (Scroll down for the link to get the Complete Project Material)

EXPERIMENTAL RESEARCH METHOD AND MATERIAL
Atmospheric Environment
The atmospheric environment in this context is a region of natural free air accessibility around the earth. Steel and other metals are more frequently exposed to the atmosphere than to any other corrosive environment. On a tonnage basis, atmospheric corrosion accounts for the greatest losses. This type of corrosion is also the oldest corrosion problem known to mankind.
The principal reason for this lies in the complexity of the variables, which determine the kinetics of the corrosion reactions. Thus corrosion rates vary from place to place, from hour to hour and from season to season. The approximate nominal composition of the atmosphere at a temperature of 38°C and pressure of 100KN/m2 as given by Meetham (1956)
Moisture: For a given atmospheric condition, corrosion will be more severe the more humid and oxygenated it is. This is why exposed and unprotected steel structures are quite durable in arid regions but rapidly attacked in humid climates. The quantity of moisture required for rusting to occur need not be great. The corrosion reactions will proceed, albeit slowly on a steel surface in such minute quantity as to be invisible… (Scroll down for the link to get the Complete Project Material)
RESEARCH FINDINGS AND DISCUSSIONS
From the foregoing discussions, it can be appreciated that different places, sites, or points around the globe have different atmospheric characteristics at different times or periods, which affect corrosion of steel, due mainly to variations in climatic conditions and levels of pollution of those places or sites. An exact detailed classification of the atmospheric environment for corrosion behavior of steel, for all times and for every location around the globe would be complex or impossible due to the complexity and variability of all the controlling factors with time and location.
Exact atmospheric conditions at a location may involve reliable instantaneous or automatic technological monitoring and analysis of the level of chemical pollution and climatic conditions of the location. However, laboratory, field, and service studies over the years have enabled engineers to identify four important influential atmospheres in corrosion behavior of steel; these are; inland rural atmospheric, inland industrial atmospheric, nominal marine atmospheric, and marine industrial atmospheric environments.
The inland rural atmospheric environment is any region of the atmosphere of more than two kilometers away from the coast that is not affected by industrial activities. These are essentially inland unpolluted environments. In other words, this environment is more or less nominal, and hence moisture containing dissolved oxygen is the primary corrosive agent. The only possible pollution of the environment could be from distant sources according to prevailing wind direction or topography… (Scroll down for the link to get the Complete Project Material)
Effect of the operating conditions:
The operating conditions have an important influence. Generally, the facts involved are complex; they include temperature, rate of flow, design features, and stray currents. The temperature of water affects the rate of rusting in several ways. First, the corrosion process shares the general tendency of chemical reactions to increase in speed with rising temperatures. More important, however, are the effects of temperature on the nature and solubility of the corrosion products.
For example, a rise in temperature will often throw down a carbonate scale; moreover, it increases the rate of diffusion of oxygen through water but decreases the solubility of this gas. Some of these effects are conflicting, with the result that under certain laboratory conditions at least the rate of rusting/temperature curve for steel immersed in the water passes through a maximum before the boiling point is reached – at about 80°C in experiments made by Friend (Friend, 1940; Laque, 1975 and Shrier, 1979).
The rate of water flow is also very important. This determines the supply of oxygen to the rusting surface and may remove corrosion products that would otherwise stifle further rusting. Plentiful oxygen supply to the cathodic area will stimulate corrosion, but so may smaller supplies at a slow rate of flow, if this leads to the formation of differential aeration cells.
At sufficiently high rates of flow in natural waters enough oxygen may reach the surface to cause partial passivity, in which case the corrosion rate may decrease. In sea-water, owing to the high concentration of chloride ions, the corrosion rate increases with velocity (m/s). In one series of tests, corrosion under static conditions was 0.125 mm/year, 0.50mm/year at 1.52m/s and 0.83 mm/year at 4.572m/s (Shreir, 1979)… (Scroll down for the link to get the Complete Project Material)
SUMMARY AND CONCLUSIONS
Corrosion is the destructive attack of a material by reaction with its environment. The serious consequences of corrosion have become a problem of worldwide significance. In addition to our everyday encounters with this form of degradation, corrosion causes plants’ shutdowns, waste of valuable resources, loss or contamination of the product, reduction in efficiency of plants or systems, costly maintenance, and expensive overdesign. Furthermore, it jeopardizes the safety and inhibits technological progress.
Corrosion control is achieved by recognizing and understanding mechanisms by using corrosion-resistant materials and designs, and by protective systems, devices, and treatments. Corrosion of steel is the most important corrosion problem in the world’s socio-economic and technological development and accounts for about 90% of all corrosion problems worldwide.
Factors that influence the corrosion of steel are complex and vary from location to location, point to point, and time to time… (Scroll down for the link to get the Complete Project Material)

REFERENCE
Vera, R., B.M. Rosales, and C. Tapia, Effect of the exposure angle in the corrosion rate of plain Steel in a marine atmosphere. Corrosion Science, 2003. 45(2): p. 321-337.
Matsunami, K., T. Kato, and K. Sugimoto, Corrosion of Steel and its estimation in an aqueous solution used in petroleum refineries. International Journal of Pressure Vessels and Piping, 1991. 45(2): p. 179-197.
Okonkwo Paul, C. and C. Adel M, Mohamed., Erosion-Corrosion in Oil and Gas Industry: A Review. International Journal of Metallurgical & Materials Science and Engineering (IJMMSE), 2014. 4(3): p. 7-28.
Han, W., et al., Characterisation of initial atmospheric corrosion Steels by field exposure and laboratory simulation. Corrosion Science, 2007. 49(7): p. 2920-2935.
Ma, Y., Y. Li, and F. Wang, Corrosion of low Steel in atmospheric environments of different chloride content. Corrosion Science, 2009. 51(5): p. 997-1006.
Mendoza, A.R. and F. Corvo, Outdoor and indoor atmospheric corrosion of non-ferrous metals. Corrosion Science, 2000. 42(7): p. 1123-1147.
Oesch, S. and M. Faller, Environmental effects on materials: The effect of the air pollutants SO2, NO2, NO, and O3 on the corrosion of copper, zinc, and aluminum. A short literature survey and results of laboratory exposures. Corrosion Science, 1997. 39(9): p. 1505-1530.
Lan, T.T.N., et al., Atmospheric corrosion of Steel under field exposure in the southern part of Vietnam. Corrosion Science, 2006. 48(1): p. 179-192.
Castaño, J.G., et al., Atmospheric corrosion of Steel in Colombia. Corrosion Science, 2010. 52(1): p. 216- 223.
Nishimura, T., et al., Electrochemical Behavior of Rust Formed on Steel in a Wet/Dry Environment Containing Chloride Ions. Corrosion, 2000. 56(9): p. 935-941.
Dugstad, A., Mechanism of Protective Film Formation During CO2 Corrosion of Steel. 1998, NACE International.
(Scroll down for the link to get the Complete Project Material)

